From Stench to Sustainability: How Sulfur is Revolutionizing Energy Storage
A world where your electric car drives twice as far on a single charge, where solar farms store energy cheaply and efficiently, and where the battery in your phone lasts days, not hours. The secret? Sulfur—yes, the same stuff that smells like rotten eggs.
At Aluminiumion.com, we love digging into the science behind futuristic tech. And sulfur batteries? They’re not just a lab experiment anymore—they’re the dark horse of the energy revolution.
🔥 Why Sulfur Batteries Are the Rockstars of Energy Storage
1. Energy Density: The Heavyweight Champion
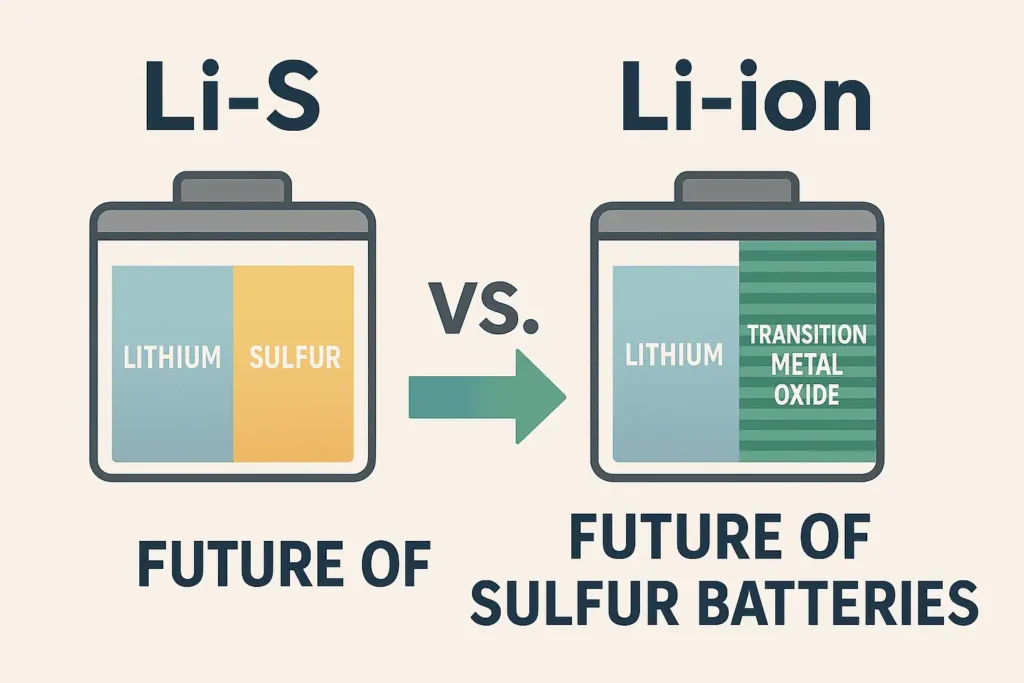
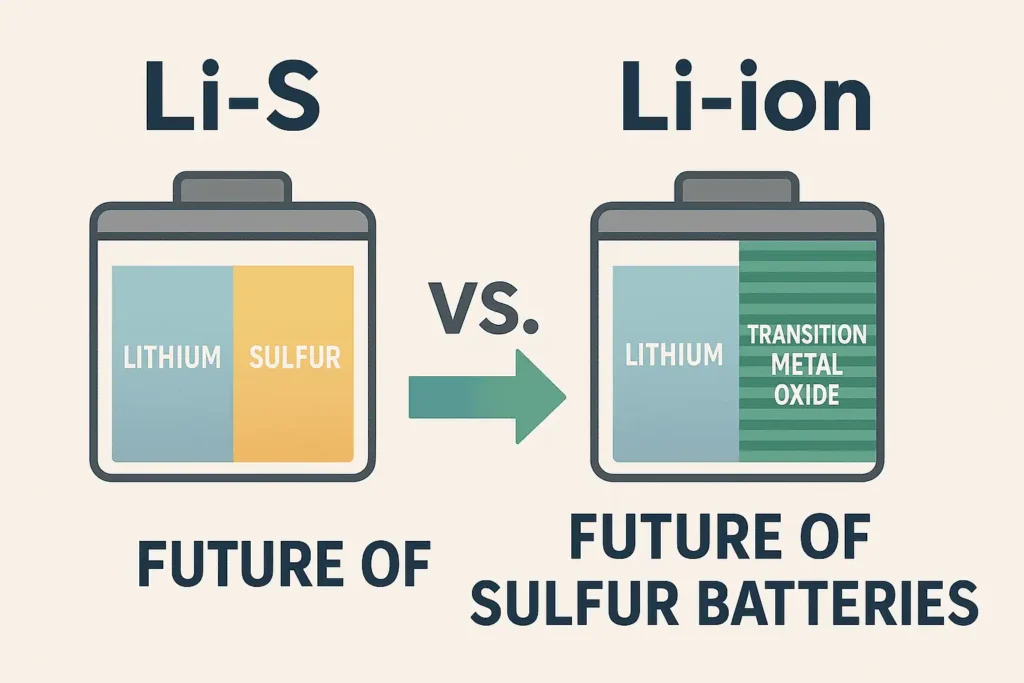
Imagine a battery that packs 3-5x more energy than today’s lithium-ion cells. That’s Lithium-Sulfur (Li-S) for you.
- Electric cars could go 1,000+ miles per charge (Tesla’s best today: ~400).
- Drones & planes could fly longer without weighing a ton.
- Smartphones might finally last a full weekend without charging.
💡 Fun Fact: If lithium-ion is a compact sedan, sulfur batteries are a hyper-efficient cargo truck—carrying way more energy per pound.
2. Dirt Cheap & Everywhere
Sulfur is one of the most abundant elements on Earth—a byproduct of oil refining.
- No need for expensive cobalt (which has ethical mining concerns).
- Costs up to 10x less than lithium-ion materials.
🌍 Bonus: Using sulfur for batteries means less waste—oil refineries produce millions of tons of sulfur yearly.
3. Greener Than a Tesla in a Solar Farm
- Non-toxic (unlike cobalt/nickel in lithium-ion).
- Fully recyclable—no nasty landfill waste.
- Safer (less risk of fiery explosions).
🚀 Future Vision: A circular economy where old batteries become new ones, endlessly.
⚡ How Do Sulfur Batteries Work? (Without the Boring Science Lecture)
Lithium-Sulfur (Li-S) vs. Lithium-Ion (Li-ion): The Ultimate Showdown
| Feature | Lithium-Sulfur (Li-S) | Lithium-Ion (Li-ion) | Winner? |
|---|---|---|---|
| Energy Density | 400-600 Wh/kg (More juice!) | 150-250 Wh/kg | 🏆 Li-S |
| Cost | $ Cheap (Sulfur is everywhere) | (Cobalt=(Cobalt=) | 🏆 Li-S |
| Lifespan | ~500 cycles (Getting better!) | ~1000+ cycles | 🏆 Li-ion |
| Eco-Friendliness | Non-toxic & recyclable | Mining concerns | 🏆 Li-S |
🔋 Sodium-Sulfur (Na-S) Batteries – The big brothers of energy storage:
- Used in massive solar/wind farms.
- Super efficient but need high temps (like a battery sauna).
🚧 The Hurdles: Why Aren’t Sulfur Batteries Everywhere Yet?
1. The “Shuttle Effect” – Sulfur’s Great Escape
Problem: Sulfur dissolves in the battery liquid, causing short lifespans.
🔬 Fix? Scientists are using:
- Graphene armor (to trap sulfur).
- Solid-state electrolytes (no leaks!).
2. Sulfur is a Terrible Conductor (Like a Broken Wire)
Solution: Mix it with super conductive carbon—like giving sulfur a turbo boost.
3. Big Companies Betting on Sulfur
- Tesla (of course) is researching it.
- Oxis Energy & Sion Power already testing prototypes.
- NASA wants it for space missions (lighter = better).
🔮 The Future: Where Will Sulfur Batteries Take Us?
1. Electric Cars That Go the Distance
Imagine:
- “Fill up” once a month.
- No more “range anxiety.”
2. Renewable Energy’s Best Friend
Solar and wind power need big, cheap storage—sulfur batteries could be the missing piece.
3. Phones That Won’t Die After 2 Hours of TikTok
📱 3-day battery life? Maybe sooner than you think.
💡 How You Can Stay Ahead of the Curve
✅ Follow Aluminiumion.com – We break down complex tech into fun, digestible insights.
✅ Share this article – Know someone into EVs or renewables? Spread the word!
✅ Watch this space – Sulfur batteries are coming faster than you think.
🎨 Interactive Element: What Would YOU Power with Sulfur Batteries?
Comment below! Which tech would you supercharge?
- 🚗 Electric cars that never need charging?
- 📱 Phones that last a week?
- 🏠 Entire cities running on solar + sulfur storage?
Final Thought: The Smelly Underdog That Could Save Energy
Sulfur batteries aren’t perfect yet—but with breakthroughs happening fast, they could dethrone lithium-ion within a decade.
Want more futuristic energy insights? Dive deeper at Aluminiumion.com!